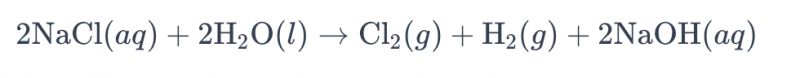Inzira yo gukoresha amashanyarazi ya brine ukoresheje electrode ya titanium kugirango ikore chlorine bakunze kwita "electrolysis ya brine." Muri iki gikorwa, electrode ya titanium ikoreshwa kugirango byorohereze okiside ya ioni ya chloride muri brine, biganisha kuri gaze ya chlorine. Ikigereranyo rusange cyimiti kubitekerezo ni ibi bikurikira:
Muri uku kugereranya, ioni ya chloride ikorerwa okiside kuri anode, bigatuma habaho gaze ya chlorine, mugihe molekile zamazi zigabanuka kuri cathode, zitanga gaze ya hydrogène. Byongeye kandi, hydroxide ion igabanuka kuri anode, ikora hydrogène na hydroxide ya sodium.
Guhitamo electrode ya titanium biterwa na titanium nziza yo kurwanya ruswa no kwifata neza, bigatuma ishobora guhinduka mugihe cya electrolysis idafite ruswa. Ibi bituma titanium electrode ihitamo neza kuri electrolysis ya brine.
Electrolysis y'amazi yumunyu mubisanzwe isaba ingufu zituruka hanze kugirango zitange ingufu za reaction ya electrolytique. Inkomoko y'amashanyarazi mubisanzwe ni amashanyarazi ataziguye (DC) kuberako reaction ya electrolytique ikenera icyerekezo gihoraho cyumuyaga, kandi amashanyarazi ya DC arashobora gutanga icyerekezo gihoraho.
Mubikorwa bya electrolyzing amazi yumunyu kugirango bibyare gaze ya chlorine, amashanyarazi ya DC afite ingufu nke. Umuvuduko w'amashanyarazi uterwa nuburyo bwihariye bwo kubyitwaramo no gushushanya ibikoresho, ariko muri rusange biri hagati ya volt 2 na 4. Byongeye kandi, ubukana bugezweho bwo gutanga amashanyarazi nikintu cyingenzi kigomba kugenwa hashingiwe ku bunini bwicyumba cya reaction ndetse n’umusaruro wifuzwa.
Muri make, guhitamo amashanyarazi kuri electrolysis yamazi yumunyu biterwa nibisabwa byihariye byubushakashatsi cyangwa inzira zinganda kugirango habeho reaction nziza no kugera kubicuruzwa byifuzwa.
Igihe cyo kohereza: Mutarama-16-2024